Ester Bron Erome - A Closer Look At Chemical Bonds
Have you ever wondered about the tiny building blocks that make up so much of what we experience every day? Think about the smells of fruits, the way certain things feel, or even some of the ingredients in your favorite lotions. Often, behind these everyday sensations, there are fascinating chemical compounds at play. One such compound, a rather important one in the world of chemistry, is called an ester. It’s a pretty neat piece of molecular architecture, and you might be surprised at how often it pops up without you even realizing it, you know?
These compounds are, in a way, like chemical chameleons, showing up in a surprising number of places and having a variety of uses. They are quite common in nature, giving many fruits and flowers their distinctive and often pleasant scents. So, the sweet smell of a banana or the lovely aroma of a rose? Very often, you're smelling an ester. They're also quite versatile, which means they can be put to work in different ways by people, too. It’s actually pretty cool how they work.
When we talk about esters, we're really getting into the fundamental ways that atoms connect and rearrange themselves to form new substances. It's a story of transformation, where one type of chemical ingredient meets another, and through a bit of a molecular dance, something entirely new comes into being. We're going to take a closer look at what these compounds are, how they come about, and what makes them so special, in a way. It’s just a little bit of chemistry for everyone.
Table of Contents
- What is an Ester, Really? Bron Erome
- How Do Esters Come to Be? Bron Erome
- What Do Esters Look Like? Bron Erome
- How Are Esters Named? Bron Erome
- The Most Common Kind of Ester, Bron Erome
- The Role of Water in Ester Reactions, Bron Erome
- Seeing the Connections, Bron Erome
- Key Things to Know About Esters, Bron Erome
What is an Ester, Really? Bron Erome
So, you might be asking yourself, what exactly is an ester in the world of chemistry? Well, it’s a type of chemical compound, which basically means it's a substance made up of two or more different elements joined together in a specific way. These compounds are, in essence, created from an acid. Now, acids themselves can come in a couple of forms; they can be what we call organic, meaning they're based on carbon, or they can be inorganic, which means they don't necessarily have that carbon backbone, you know?
The way an ester forms is pretty interesting. Imagine an acid molecule has a particular spot, a sort of special grouping of atoms, that includes a hydrogen atom. This specific part is often called an acidic hydroxyl group. What happens when an ester forms is that this hydrogen atom, this tiny little piece, gets swapped out. It's replaced by something else, another chemical piece, which then connects itself to that spot. It’s almost like a puzzle piece being exchanged for a different one, but it fits perfectly, anyway.
This replacement is a key part of what makes an ester, well, an ester. It changes the nature of the original acid, giving it new properties and characteristics. So, while it starts with an acid, the end result, the ester, behaves differently and has its own unique set of traits. It’s a fundamental concept in how many different compounds are built up in chemistry, which is pretty neat to consider, you know? It's a basic building block, in some respects.
Think of it this way: you have a basic ingredient, the acid, and then you modify it slightly by taking out one small part and putting in another. This small change has a big impact on what the final compound is like. It’s a bit like taking a basic recipe and swapping out one key ingredient to create a completely different dish. The essence is there, but the flavor, or in this case, the chemical behavior, is transformed, that is.
How Do Esters Come to Be? Bron Erome
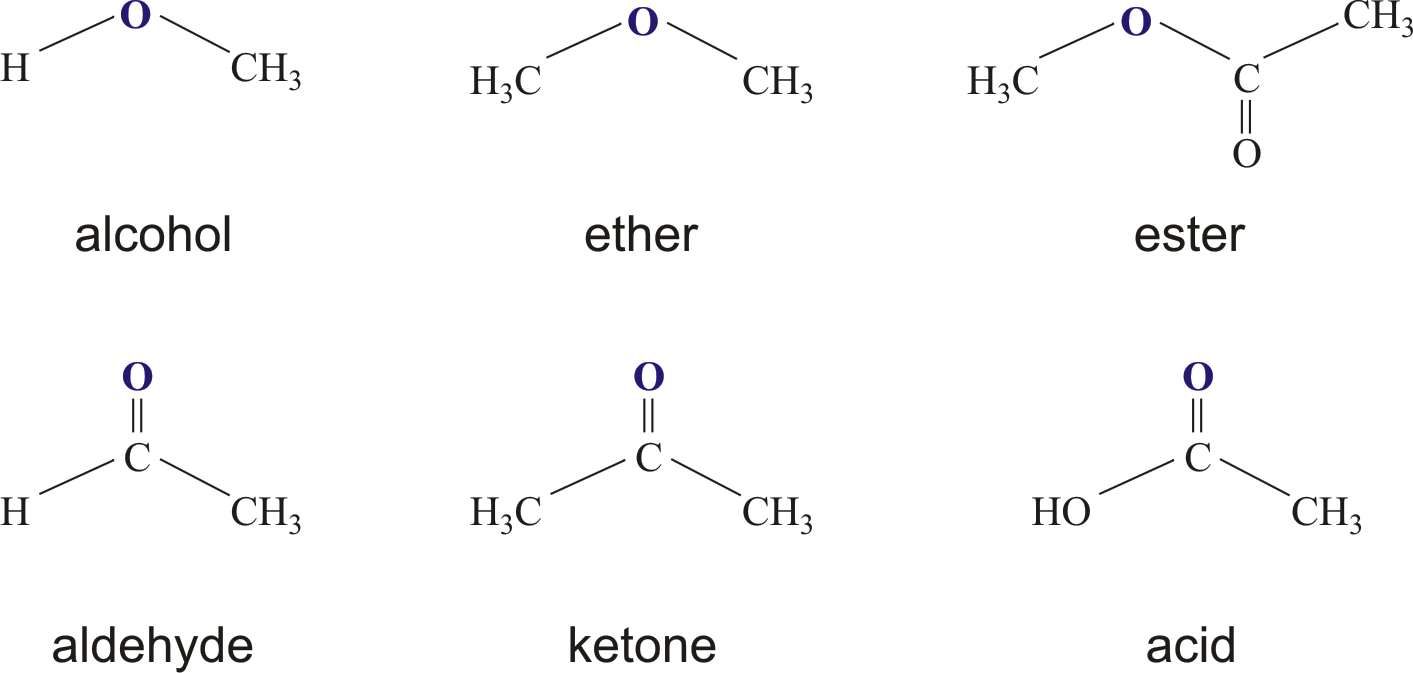
Esters are a type of organic compound, which means they contain carbon atoms, and they have a rather specific way of forming. They are created when two different kinds of molecules come together and react. One of these molecules is an alcohol, and the other is an acid. It’s a sort of chemical partnership that results in something new, you know?
The process often involves what chemists call a condensation reaction. This sounds a bit technical, but what it basically means is that when the alcohol and the acid join up, a small molecule of water is actually removed, or lost, from the combination. So, it’s not just a simple joining; there’s a byproduct, which is water. It’s kind of like building something and having a few leftover pieces that aren't part of the main structure, that is.
This simultaneous loss of water is a very important part of the reaction. It’s what allows the alcohol and the acid to link together in a particular way, forming the characteristic structure of an ester. Without that water being removed, the connection wouldn't happen in the same manner. It's a pretty elegant process when you think about it, allowing for the creation of a wide variety of these compounds, you know?
So, to put it simply, an ester is born when an alcohol and an acid decide to get together, and in the process, they kick out a water molecule. This union creates a new compound with its own set of properties, which are often quite different from the original alcohol and acid. It’s a very common and important reaction in organic chemistry, actually, and it helps explain how many natural and man-made substances are formed, that is.
What Do Esters Look Like? Bron Erome
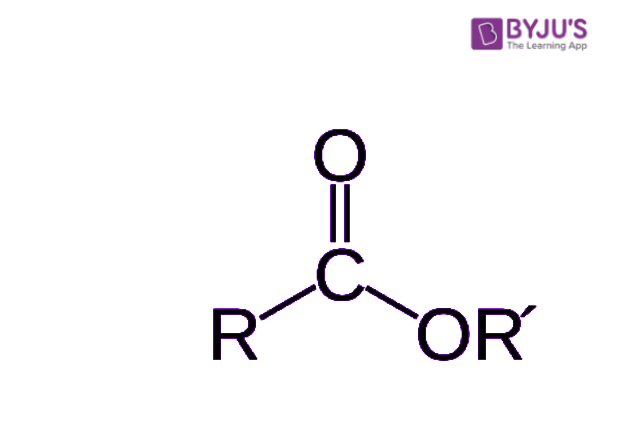
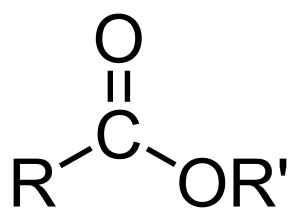
When we talk about what an ester "looks like" in chemistry, we're really talking about its general structure, or how its atoms are arranged. Every ester has a particular pattern of atoms that makes it identifiable. There's a central part, a kind of core, where a carbon atom is double-bonded to one oxygen atom and also single-bonded to another oxygen atom. This second oxygen atom then connects to another carbon atom, you know?
This specific arrangement of carbon and oxygen atoms is what gives an ester its unique chemical identity. It's like a signature pattern that all esters share, even though the parts attached to this core can be very different. So, while one ester might have a long chain of carbon atoms attached, and another might have a shorter, more branched chain, that central arrangement remains the same. It’s pretty consistent, in a way.
If you were to look at a diagram of an ester, you'd clearly see this distinctive grouping. The way the second oxygen atom connects to another carbon atom is a key feature. This connection is what helps define the overall shape and behavior of the ester molecule. It's a bit like looking at a blueprint; certain sections are always present, even if the surrounding parts change, that is.
Understanding this basic structure is a fundamental part of learning about esters. It helps you predict how they might react with other substances and gives you clues about their physical properties, like whether they're liquids or solids, or what they might smell like. It's really about recognizing that core pattern that defines them as a class of compounds, you know? It's a very important visual cue, apparently.
How Are Esters Named? Bron Erome
Giving names to chemical compounds might seem like a complicated task, but there's actually a system to it, especially when it comes to esters. When you're trying to figure out what an ester is called, or if you're trying to name one yourself, there are a couple of ways people typically do it. One way involves using what are called common names, which are often simpler and more widely used in everyday conversation or older texts, you know?
However, for a more precise and universally understood way of naming, chemists rely on a system developed by the International Union of Pure and Applied Chemistry, often just called IUPAC. This system provides a set of rules that ensures everyone, no matter where they are in the world, can understand exactly which compound is being discussed based on its name. It’s a very standardized approach, which is quite helpful, actually.
When you name an ester using either of these systems, the name itself often gives you a lot of information about the molecule's structure. For instance, the names for esters usually include specific prefixes. These little bits added to the beginning of the name are there to tell you about the lengths of the carbon chains within the molecule. So, just by hearing the name, you can get a pretty good idea of how big or small certain parts of the ester are, that is.
It’s a bit like how we name streets; the name often tells you something about its location or what it connects to. In the case of esters, the naming conventions are designed to convey structural information, making it easier to visualize the compound and understand its properties. So, learning how these names work is a pretty important step in getting to know esters better, you know? It's a very practical system, apparently.
The Most Common Kind of Ester, Bron Erome
While there are many different types of esters out there, some are much more frequently encountered than others. The esters that you hear about most often, or the ones that are very common in both natural settings and in industrial applications, actually come from a particular kind of acid. These are known as carboxylic acids, and they are a very important family of organic compounds themselves, you know?
So, to be a bit more specific, an ester is an organic compound where a hydrogen atom, which is usually found in a specific part of the acid called the carboxyl group, gets replaced. Instead of that hydrogen, a hydrocarbon group takes its place. A hydrocarbon group is just a piece of a molecule made up of carbon and hydrogen atoms. This switch is what turns a carboxylic acid into a carboxylic ester, which is a key distinction, that is.
This means that when we talk about esters generally, we are very often referring to those that have been derived from carboxylic acids. They are the most prevalent and widely studied type, and they form the basis for many of the esters we encounter in our daily lives. Think about the flavors and fragrances in food and cosmetics; many of those are carboxylic esters. It's a pretty broad category, actually.
The relationship between carboxylic acids and these esters is fundamental. It’s a direct derivation, meaning one comes directly from the other through that specific chemical reaction we talked about earlier, where water is removed. So, if you understand carboxylic acids, you’re already well on your way to understanding the most common kind of ester, which is quite helpful, you know? It's a very direct lineage, apparently.
The Role of Water in Ester Reactions, Bron Erome
When we discuss how esters are formed, or even how they can break apart, water plays a surprisingly important role. It's not just a passive observer in these chemical processes; it's an active participant, you know? As we touched on earlier, an ester is often created through a process where an alcohol and an acid combine, and at the same time, a molecule of water is essentially pushed out of the reaction. This is what we call a condensation reaction, and it’s a very common way that more complex molecules are built up from simpler ones.
But the relationship with water doesn't stop there. Esters also have the ability to react with water in a process that is, in a way, the reverse of their formation. When an ester comes into contact with water under the right conditions, it can actually break down. This breaking apart process is called hydrolysis, and it causes the ester to split back into the alcohol and the acid from which it was originally formed. It’s a bit like un-doing a chemical bond, you know?
This ability to react with water, both in their formation and their breakdown, highlights the dynamic nature of esters. They are not static compounds; they can be created and then, under different circumstances, revert back to their original components. This is actually quite useful in many chemical processes, allowing for the synthesis of new compounds and then, if needed, their decomposition. It’s a very versatile chemical relationship, apparently.
So, whether it's forming an ester by removing water or breaking an ester apart by adding water, the interaction with H2O is central to their chemistry. It’s a fundamental aspect of how these compounds behave and how they can be manipulated in various applications, from making soaps to creating flavors. It’s just a little bit of how water influences these tiny chemical building blocks, that is.
Seeing the Connections, Bron Erome
Sometimes, understanding chemistry really clicks when you can see the relationships between different compounds. It's one thing to talk about acids and alcohols and esters separately, but it’s another thing entirely to see how they are directly connected, you know? For instance, if you were to look at a chemical diagram, it would often show you a clear picture of how ethanoic acid and ethanol, which is a type of alcohol, are linked to the ester that forms from them. It truly helps to visualize how these pieces fit together.
This kind of visual representation makes the concept of chemical reactions much more tangible. You can literally trace the atoms and see how they rearrange themselves from the starting materials, the acid and the alcohol, to form the new product, the ester. It’s not just abstract ideas; it’s about tangible molecular transformations. It helps you grasp the flow of the reaction, in a way, and understand why certain products are formed, that is.
Such diagrams are invaluable tools for anyone trying to grasp these concepts. They illustrate the specific atoms involved, the bonds that are broken, and the new bonds that are formed during the creation of an ester. They provide a clear, concise way to communicate complex chemical information, making it easier to learn and remember. It’s almost like a map for molecules, you know, showing you the path from one compound to another, that is.
So, whenever you encounter a discussion about esters, remember that there’s usually a visual aid that can really bring the chemistry to life. Seeing the relationship between the starting acid, the alcohol, and the resulting ester can make all the difference in truly grasping how these fascinating compounds come into being. It’s a very helpful learning tool, apparently, for understanding these chemical connections.
Key Things to Know About Esters, Bron Erome
When you're trying to get a good handle on esters, there are a few main things that are really important to keep in mind. One of the primary objectives in learning about them is to be able to identify their general structure. This means recognizing that characteristic arrangement of carbon and oxygen atoms that defines an ester, no matter what other groups are attached to it. It’s like knowing the basic shape of a key that will open many different locks, you know?
Another crucial aspect is understanding how to name these compounds. As we discussed, there are common names, which are often simpler, and then there's the more formal IUPAC system, which provides a precise, universally recognized name based on the ester's exact structure. Being able to use both, or at least understand them, is quite helpful. The name itself often gives clues about the molecule's makeup, which is pretty neat, actually.
It's also really important to remember that esters are typically formed from acids, especially carboxylic acids, and alcohols. This fundamental reaction, often involving the removal of water, is at the heart of their creation. Knowing this relationship helps you understand where esters come from and how they fit into the broader family of organic compounds. It’s a very foundational concept, that is.
Finally, keep in mind that esters are a class of organic compounds that, when they react with water, can break down into alcohols and acids. This reverse reaction, called hydrolysis, is just as important as their formation. So, in essence, understanding esters means grasping their structure, how they're named, how they're made, and how they can be unmade. It’s a pretty comprehensive picture, you know, of these versatile chemical players.
Ester - Definition, Structure, Esterification along with Properties & Uses
Ester @ Chemistry Dictionary & Glossary
Ester Definition, Examples And Facts | Chemistry Dictionary